Lancet Oncology study
ERN GENTURIS study published in Lancet Oncology highlights high risk mutations associated with the development of Hereditary Diffuse Gastric Cancer related cancers
Picture by Ana Rita Barbosa de Matos, i3S, Porto
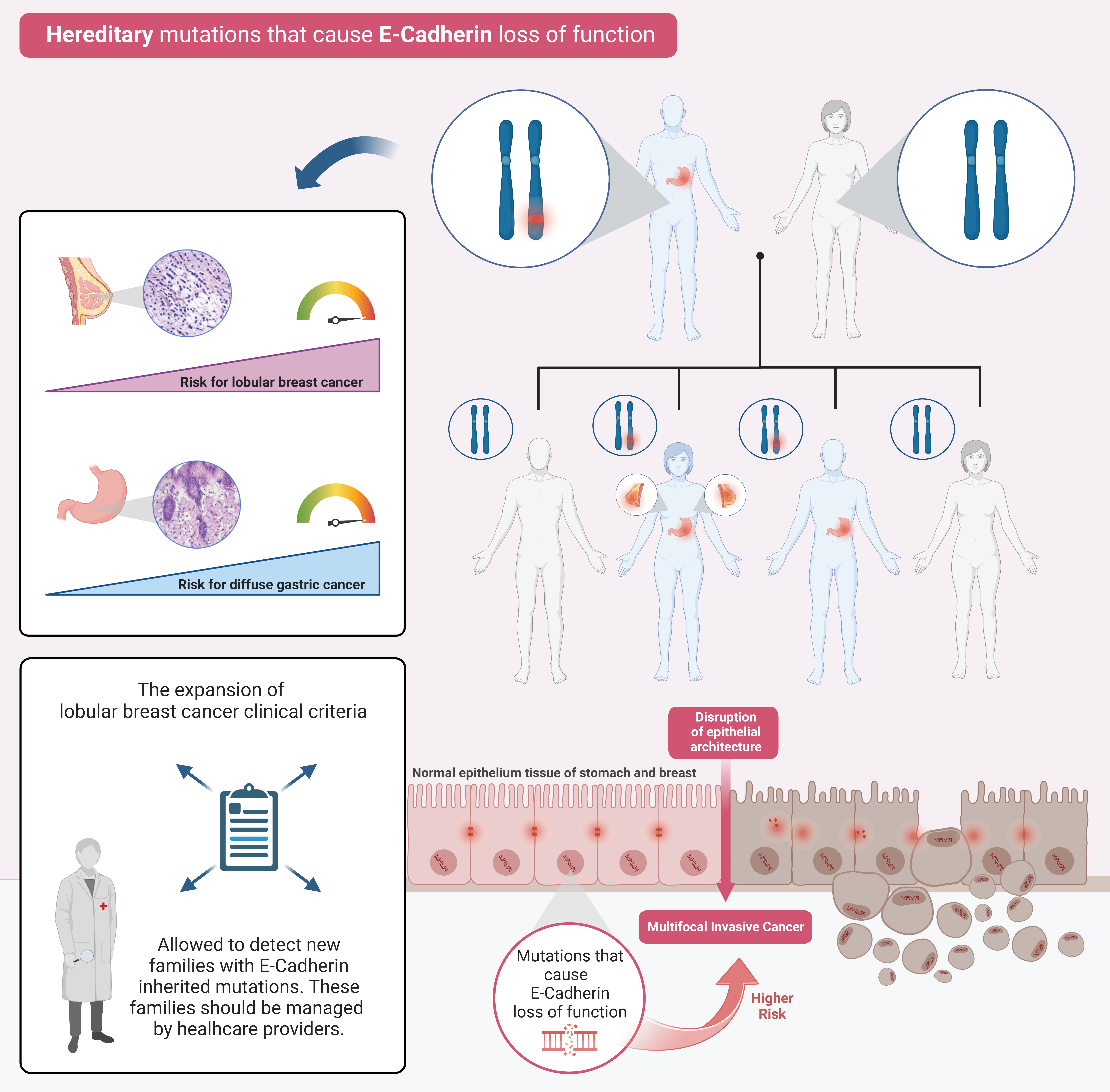
An international team, led by Carla Oliveira, from the Institute for Research and Innovation in Health of University of Porto (i3S), published a study in the Lancet Oncology journal identifying the alterations in the CDH1 gene that specifically increase the risk of developing cancers associated to Hereditary Diffuse Gastric Cancer (HDGC) syndrome. This study has also defined three new clinical criteria, in addition to those currently used, which will be fundamental to identify families at risk for genetic testing, and to act prophylactically in order to prevent the development of these oncologic diseases of extremely high mortality.
It is known that the CDH1 gene, which encodes the protein Cadherin-E, is closely linked to the development of the Hereditary Diffuse Gastric Cancer (HDGC) syndrome. In this work, the international team of experts from the European Reference Network GENTURIS studied various types of variants occurring in this gene and proved that «only the changes that eliminate the production of Cadherin-E increase the risk of developing breast cancer and stomach cancer», explains the leading scientist Carla Oliveira. There are other alterations in the same gene, namely those that only change one amino acid in the CDH1 gene protein, but these do not increase the risk of developing these oncologic diseases.
This clarification of the E-Cadherin gene variants that confer a greater propensity to develop Hereditary Diffuse Gastric Cancer (HDGC) related cancers, and which types of cancer are more prevalent in carriers of these changes, adds José Garcia-Pelaez, first author of the article, and also from i3S, "allows us to rethink the clinical criteria for genetic testing, in order to improve the clinical management of families carrying these variants".
The work now published, highlights Carla Oliveira, is the first study that relates different types of CDH1 variants with clinical data from real patients with Hereditary Diffuse Gastric Cancer (HDGC) syndrome. "It was a multicentric work, with data from 29 laboratories from 10 European countries, which included 854 carriers of 398 different rare variants in the CDH1 gene, as well as over a thousand relatives of these carriers who were clinically followed and developed about 2000 cancers," explains Carla Oliveira.
To be able to carry out this large-scale study, the team led by the i3S scientist, used information from genetic tests and clinical data available at the European Reference Network on Risk Tumour Syndromes (ERN-GENTURIS), whose Portuguese representative is the researcher Carla Oliveira. "Clarifying the specific mutations in the CDH1 gene that constitute a real risk for the individual and which mutations raise no concern, allowed us to advance with three new clinical criteria, in addition to those currently used, that will increase the number of families at risk with a CDH1 positive genetic test " reveals José Garcia-Pelaez.
"There are families with a heavy history of breast cancer that do not meet current clinical criteria for CDH1 testing, but indeed carry mutations in the CDH1 gene, and are at increased risk for this syndrome". The conclusions of this study, underlines Carla Oliveira, «allow us to propose more assertive clinical criteria that maximize the identification of families at risk for this syndrome". These new clinical criteria will be validated among peers in the next meeting of the consortium that gathers international experts in this disease, which will take place in Porto, in the spring of 2024.